Alivira's Vet APIs
Albendazole
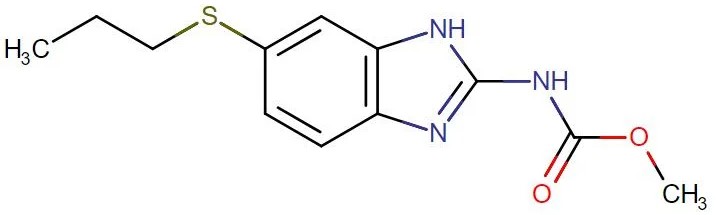
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, USDMF, CEP
CAS ID:
54965-21-8
Clorsulon
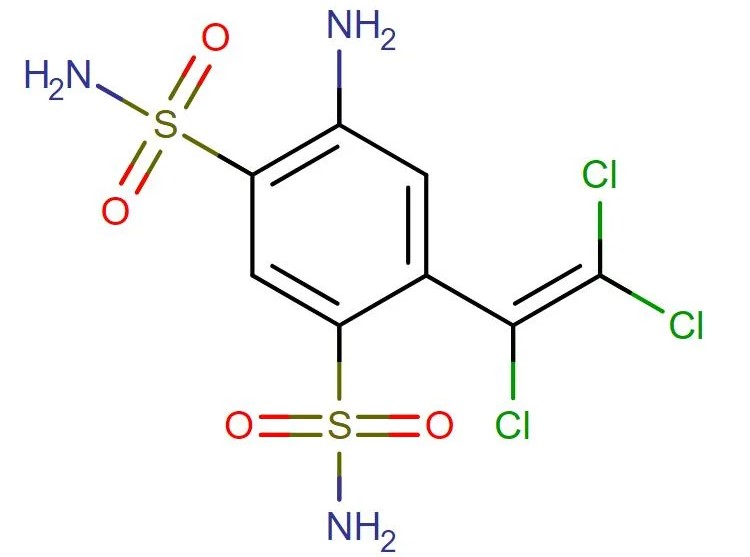
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, ASMF
CAS ID:
60200-06-8
Closantel sodium
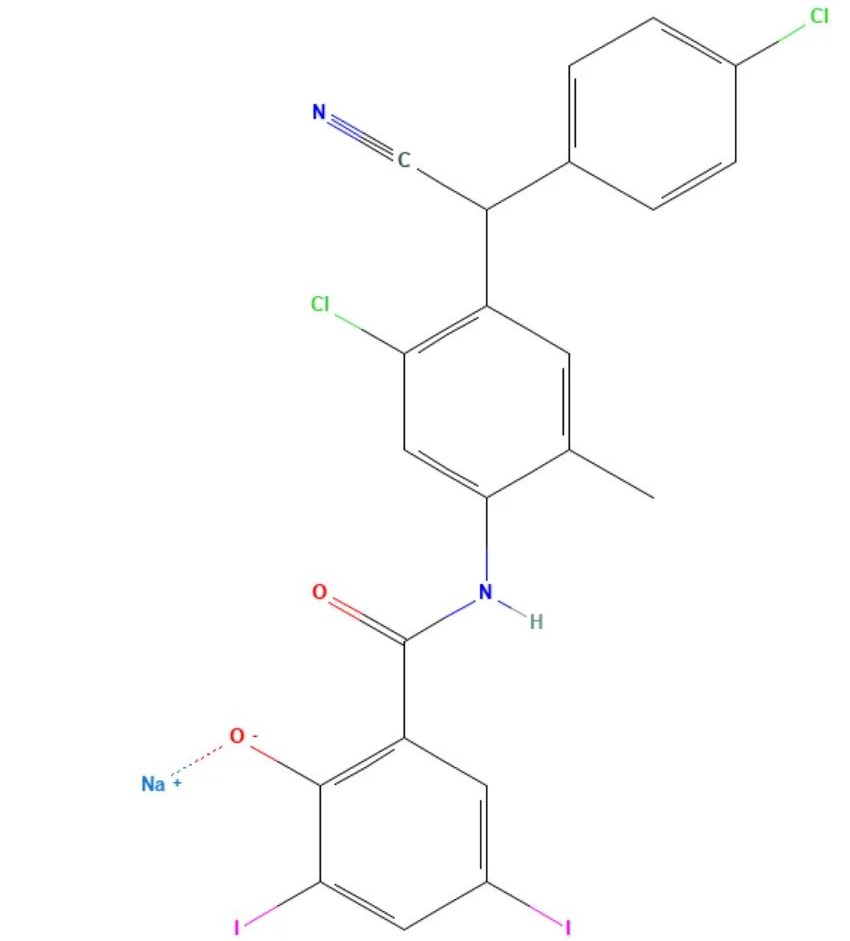
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
61438-64-0
Fenbendazole
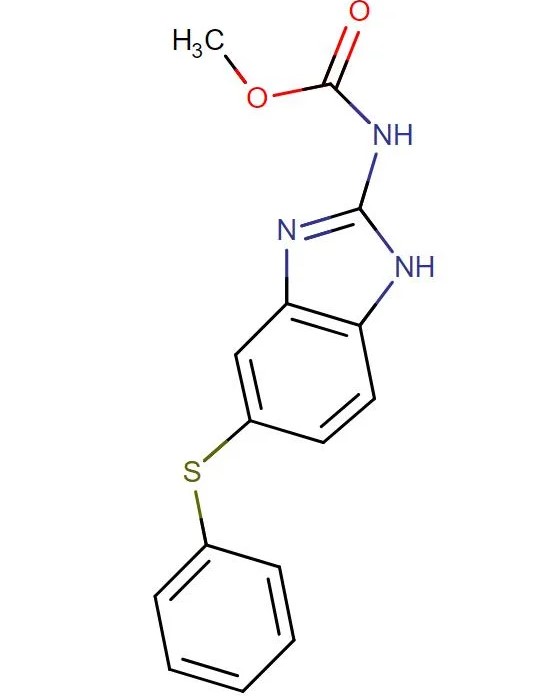
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP
CAS ID:
43210-67-9
Febantel
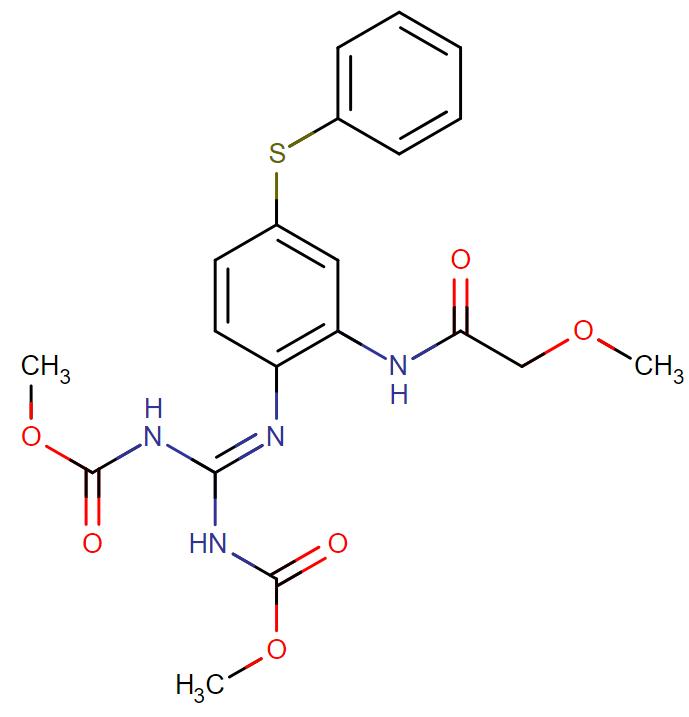
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP
CAS ID:
58306-30-2
Flubendazole
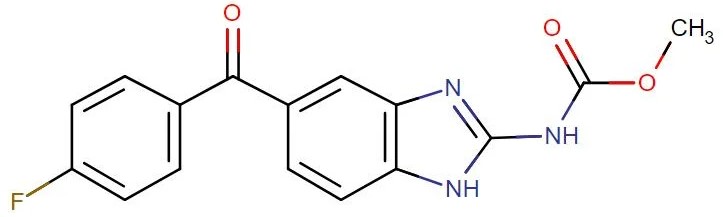
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP (under review)
CAS ID:
31430-15-6
Levamisole HCL
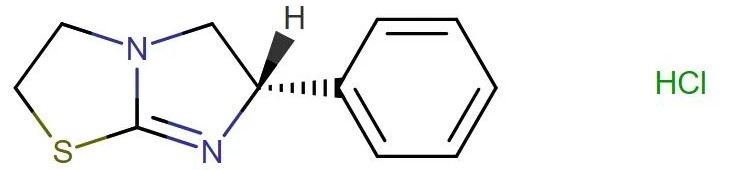
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
16595-80-5
Levamisole Base/Phosphate
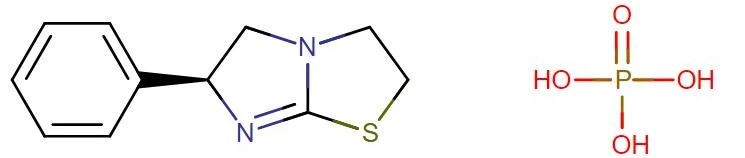
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
32093-35-9
Nitroxynil
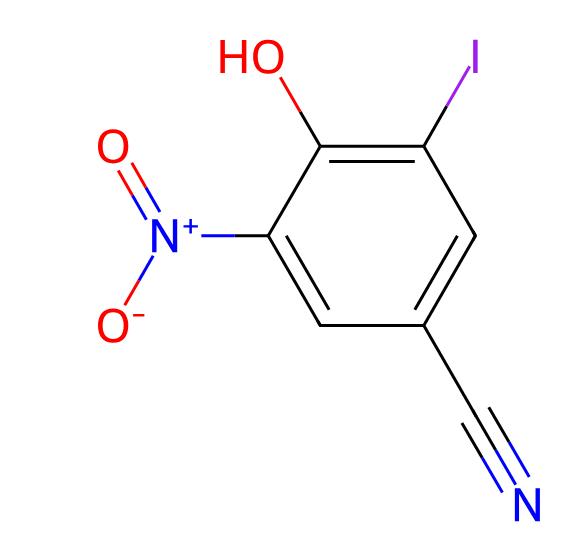
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, ASMF
CAS ID:
1689-89-0
Nitroscanate
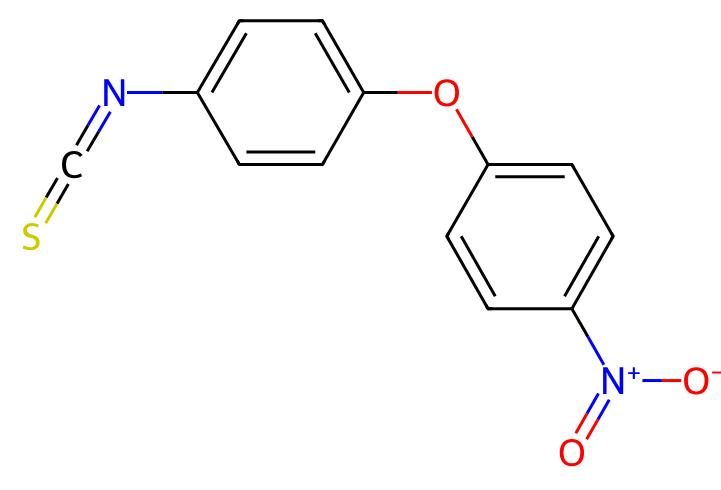
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
ASMF
CAS ID:
19881-18-6
Oxfendazole
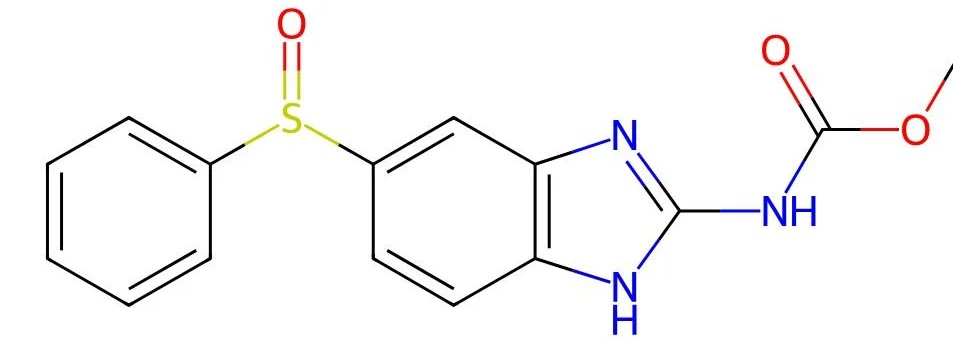
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP
CAS ID:
53716-50-0
Praziquantel
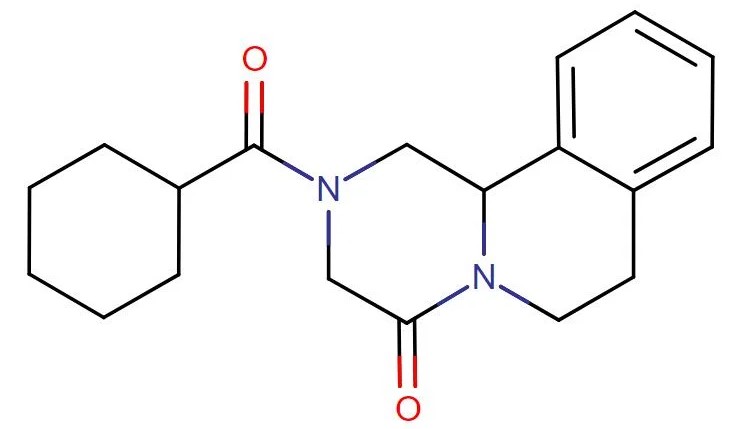
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP
CAS ID:
55268-74-1
Ricobendazole
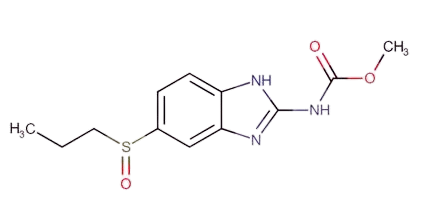
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
54029-12-8
R-praziquantel
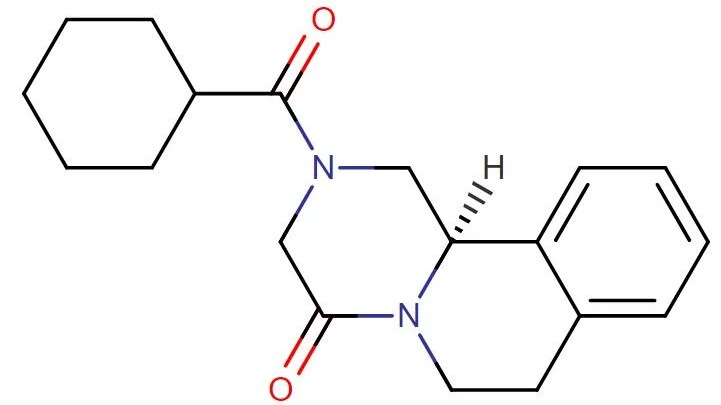
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
57452-98-9
Tetramisole HCL
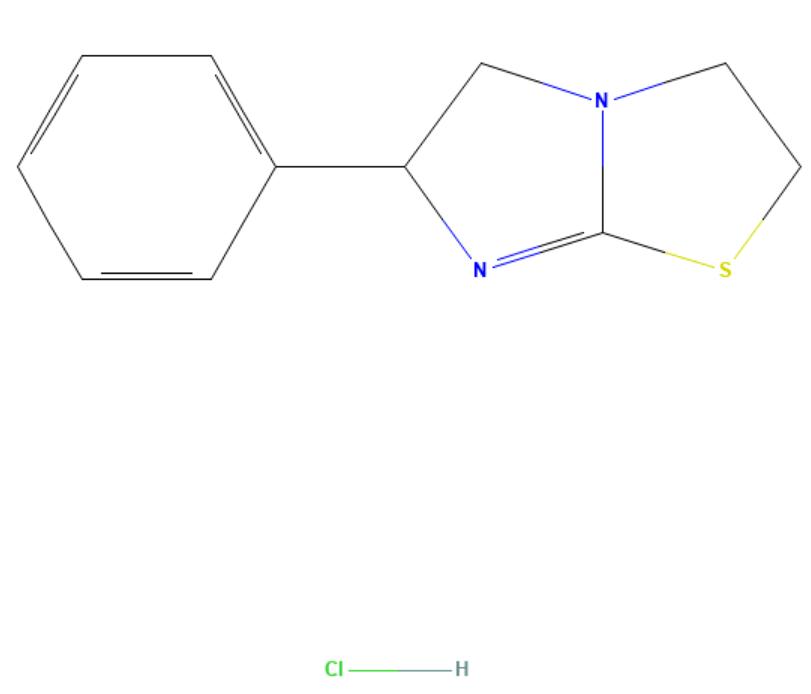
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
5086-74-8
Triclabendazole
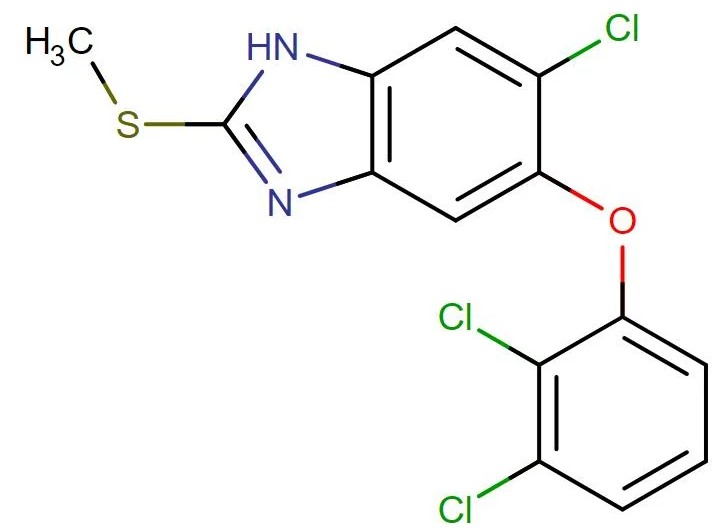
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, USDMF, CEP
CAS ID:
68786-66-3
Afoxolaner
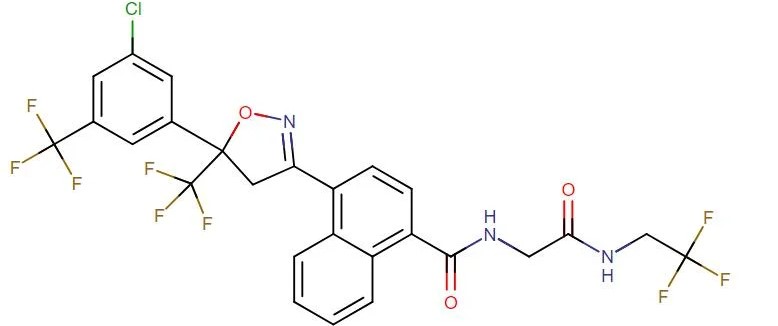
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
To be filed (under preparation)
CAS ID:
1093861-60-9
Esafoxolaner
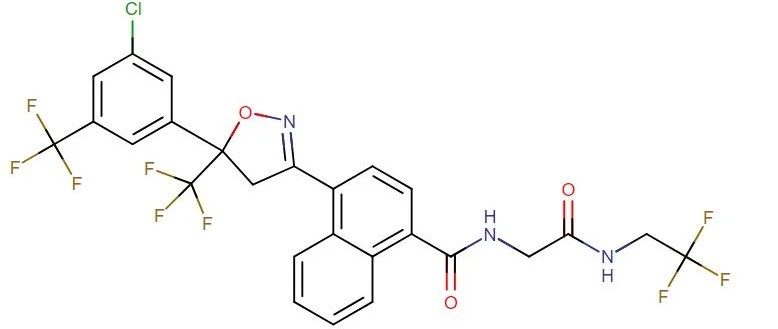
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
1093861-60-9
Fluralaner
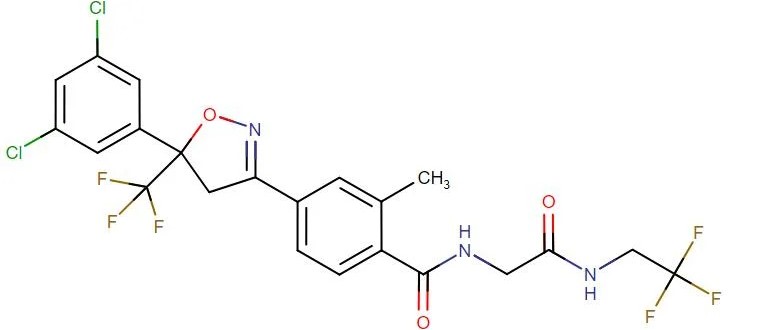
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
864731-61-3
Fluazuron
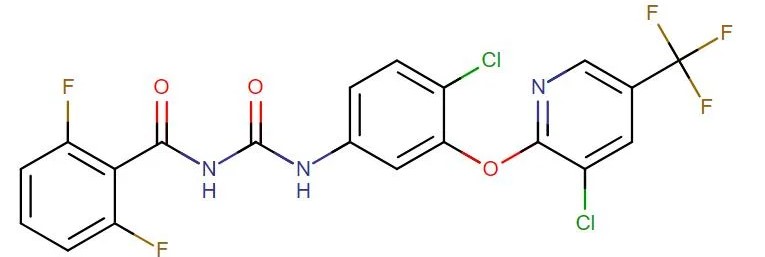
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
86811-58-7
Lotilaner
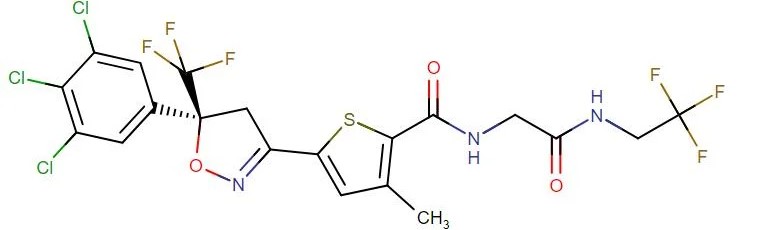
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
1369852-71-0
Lufenuron
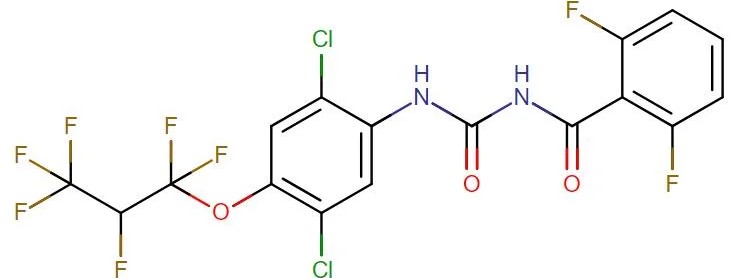
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP filed (Approval pending)
CAS ID:
103055-07-8
Sarolaner
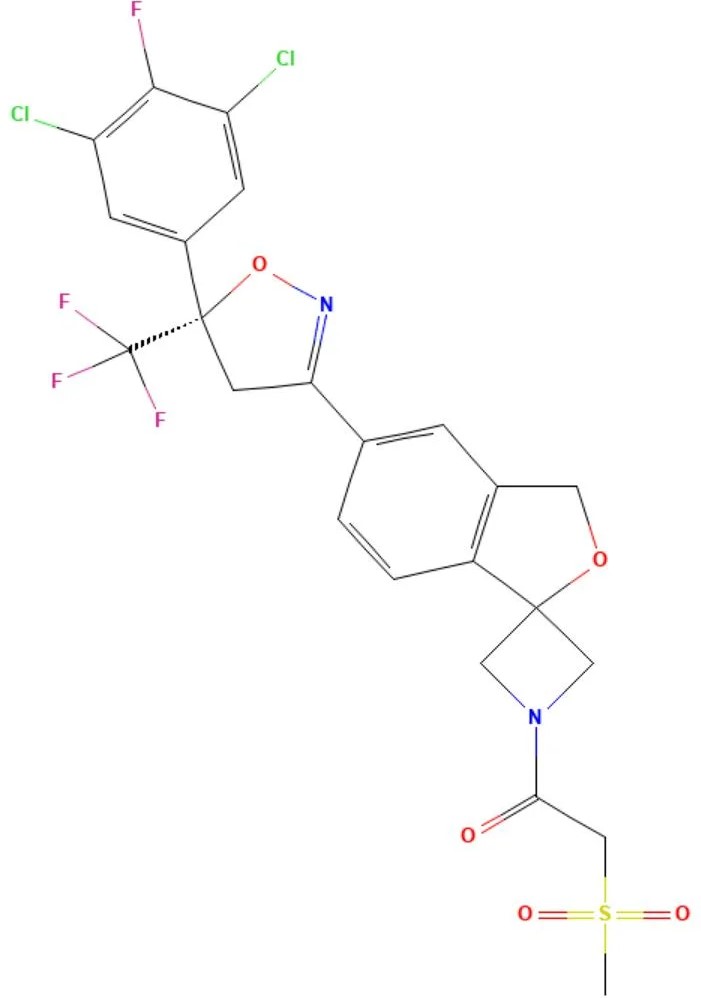
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
1398609-39-6
S-methoprene
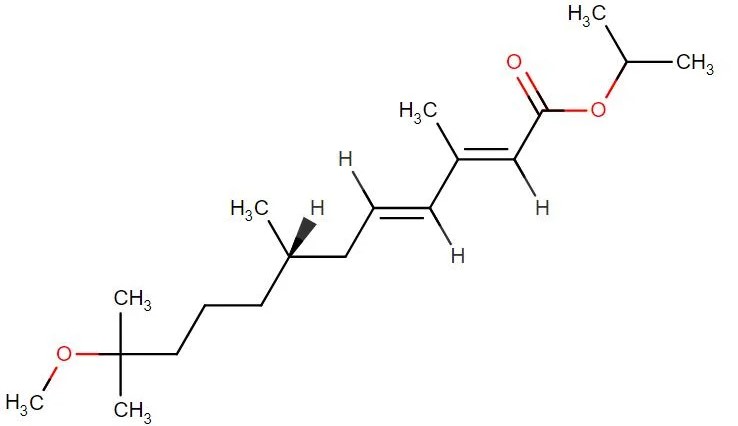
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
ASMF
CAS ID:
65733-16-6
Tigolaner
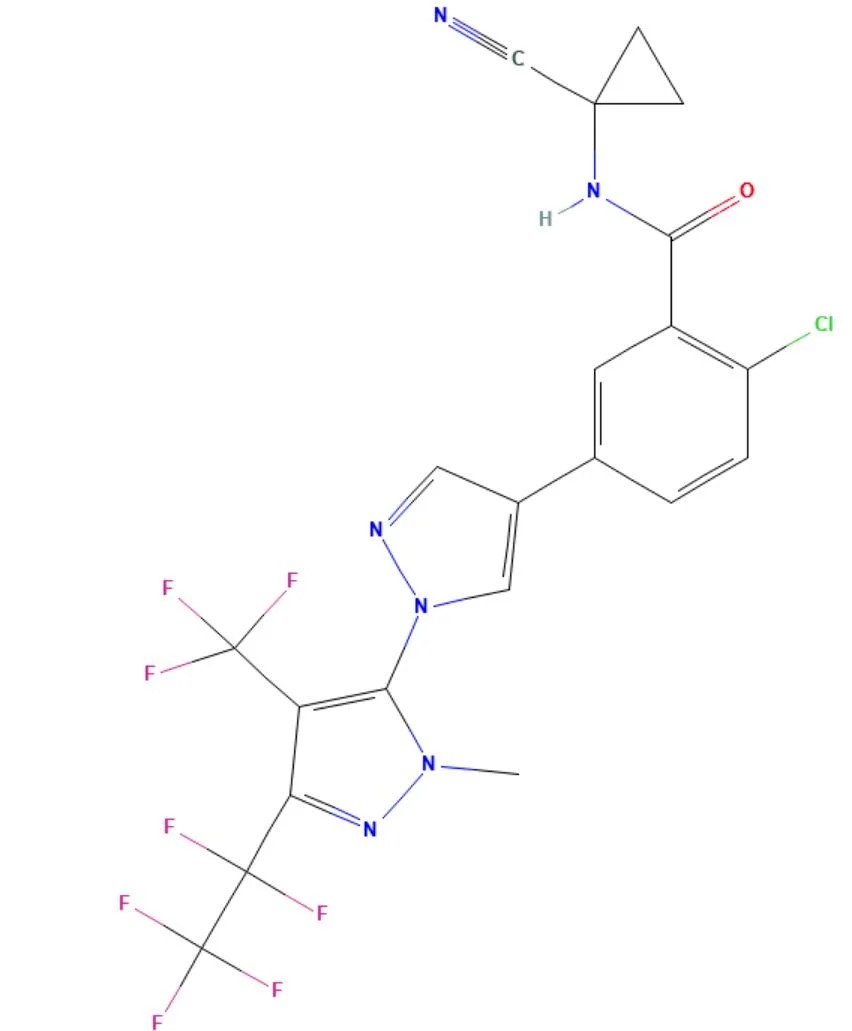
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
1621436-41-6
Buparvaquone
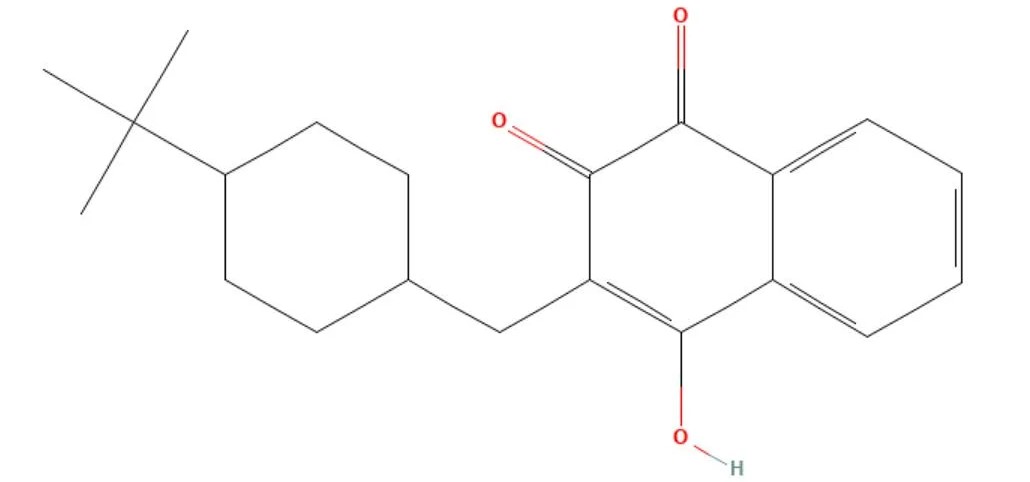
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
88426-33-9
Diclazuril
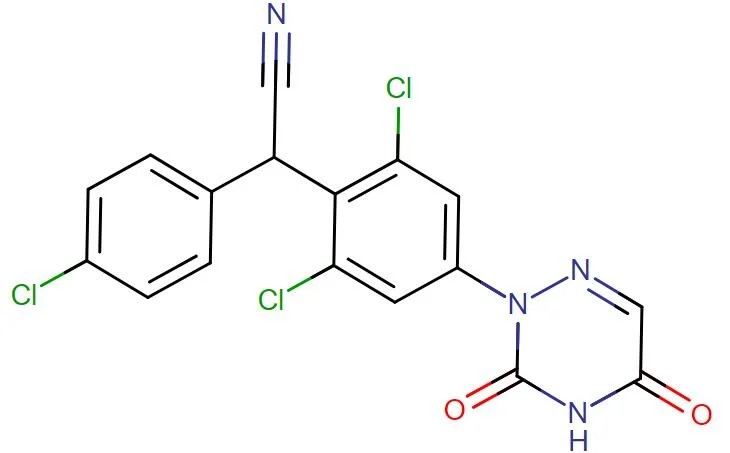
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP
CAS ID:
101831-37-2
Diminazene diaceturate
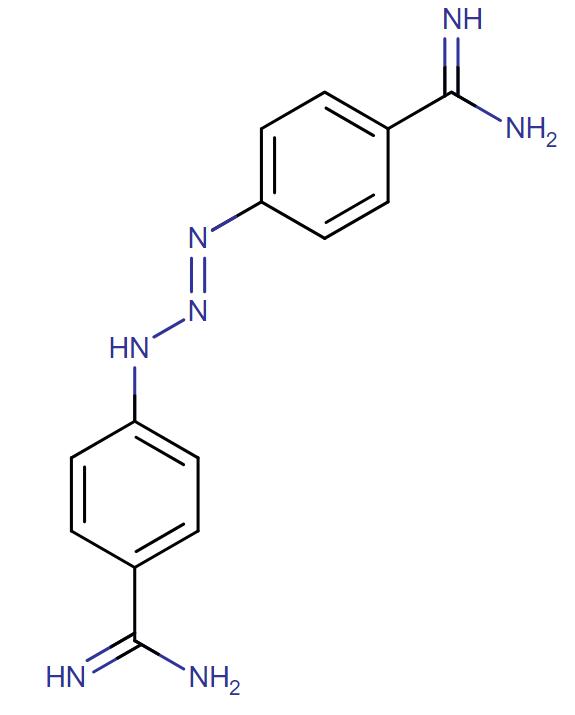
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
908-54-3
Imidocarb dipropionate
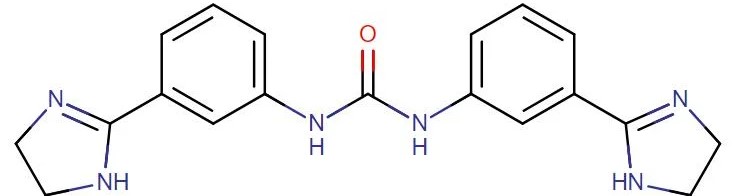
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, ASMF
CAS ID:
55750-06-6
Ponazuril
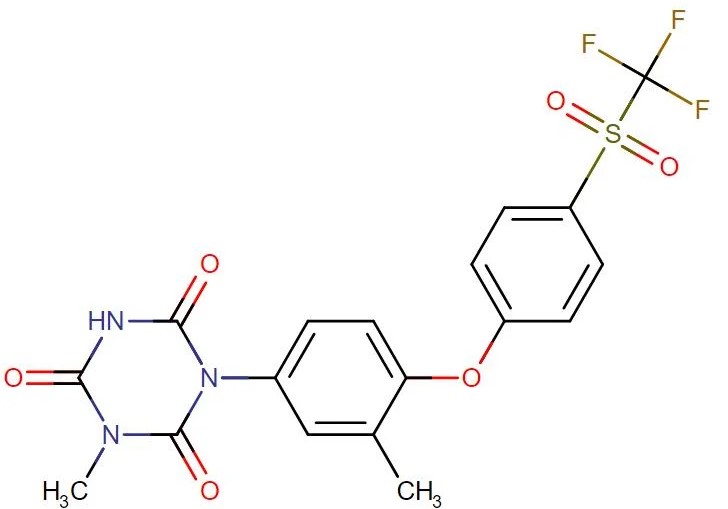
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
69004-04-2
Parvaquone
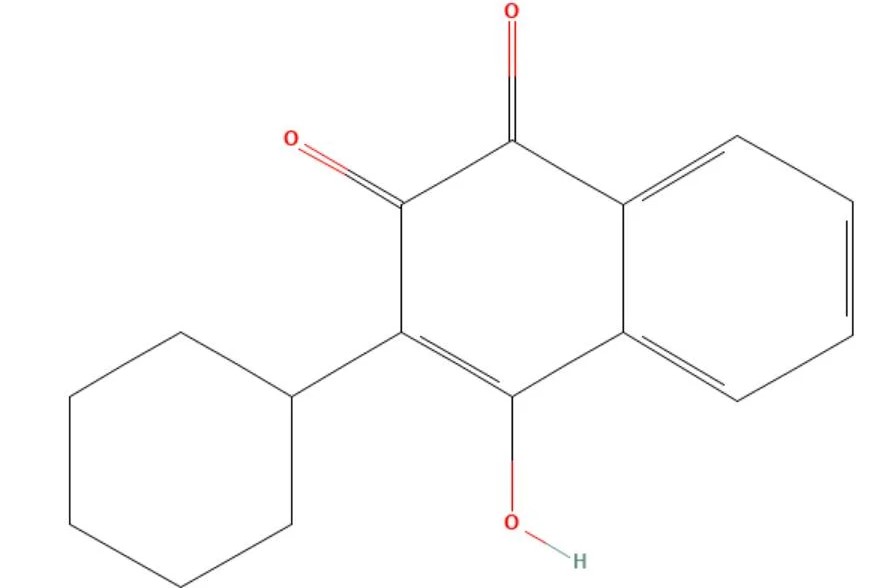
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
4042-30-2
Toltrazuril
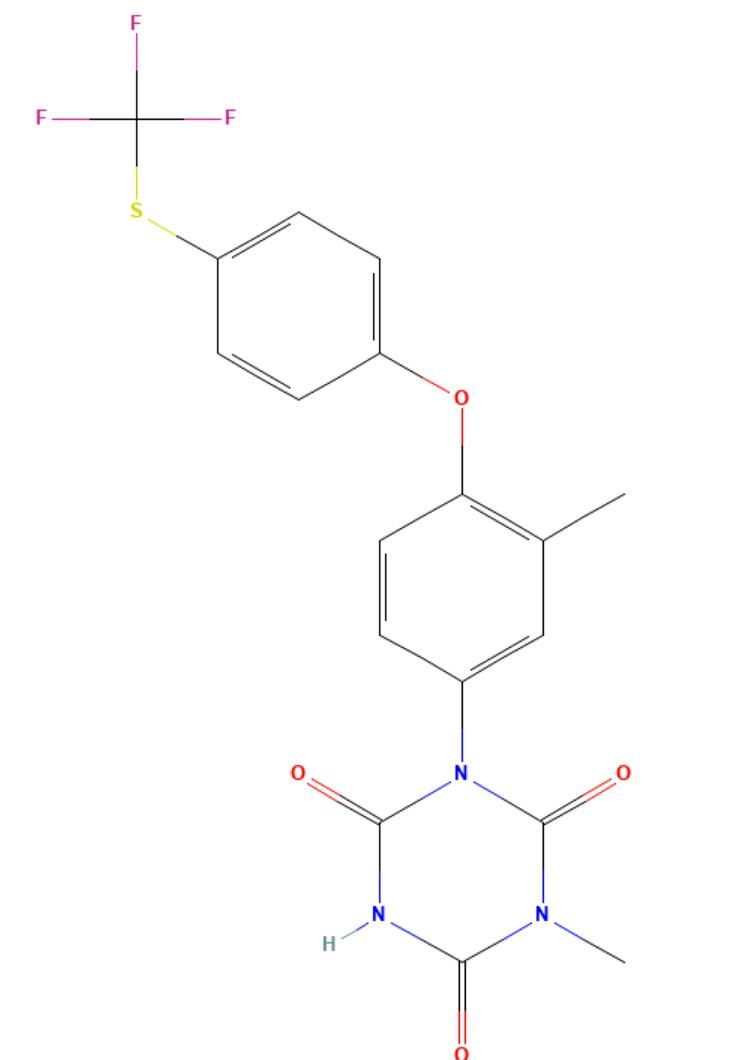
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
69004-03-1
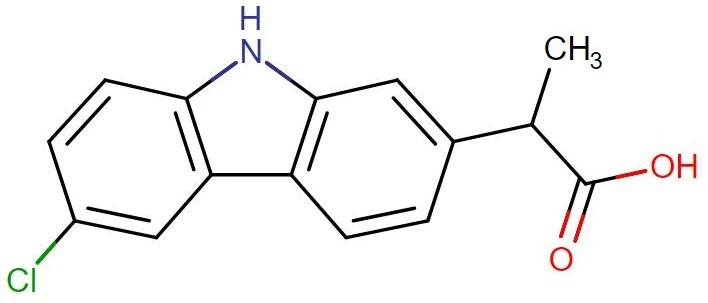
Carprofen
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP
CAS ID:
53716-49-7
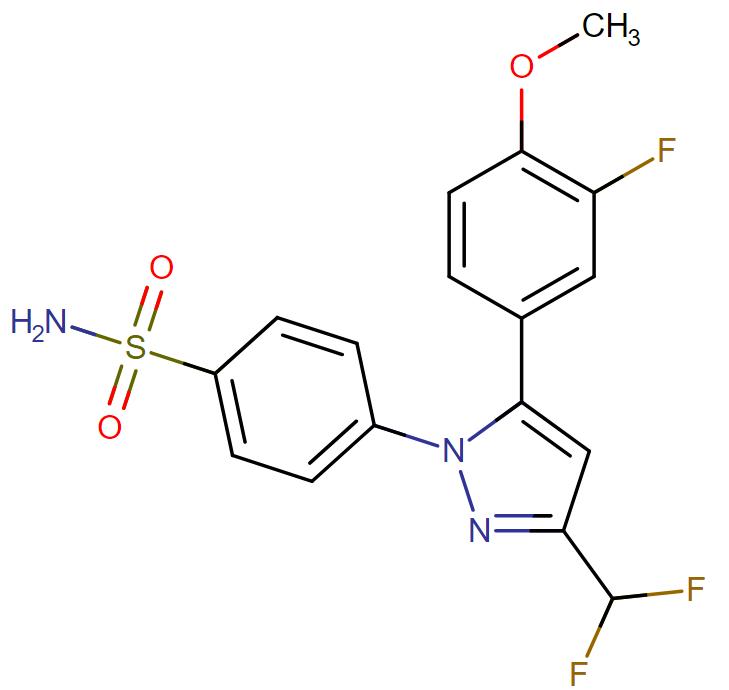
Deracoxib
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
169590-41-4
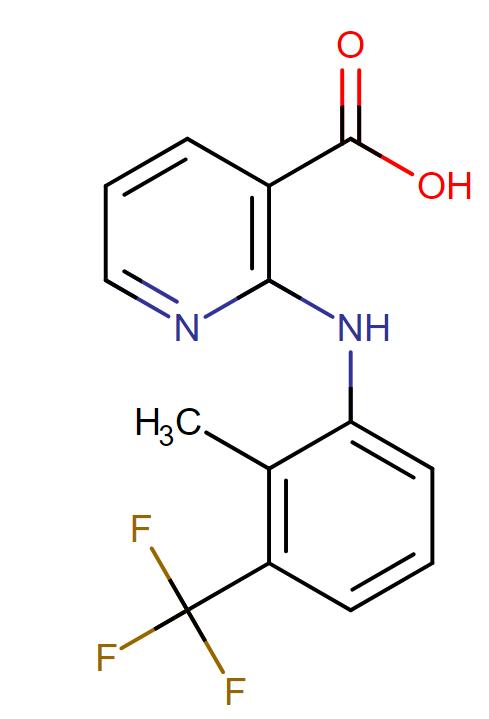
Flunixin Meglumine
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP
CAS ID:
42461-84-7
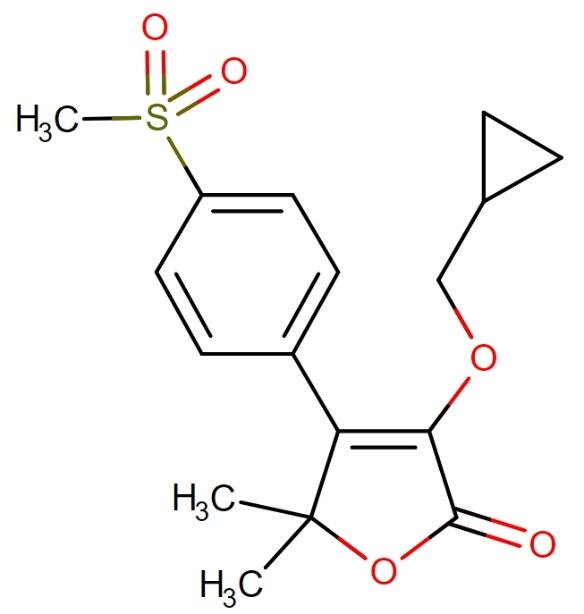
Firocoxib
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, ASMF
CAS ID:
189954-96-9
Grapiprant
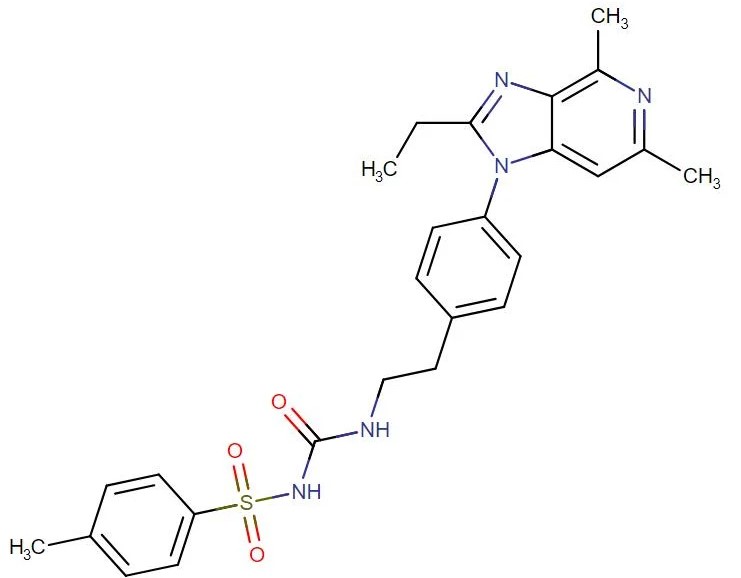
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
415903-37-6
Mavacoxib
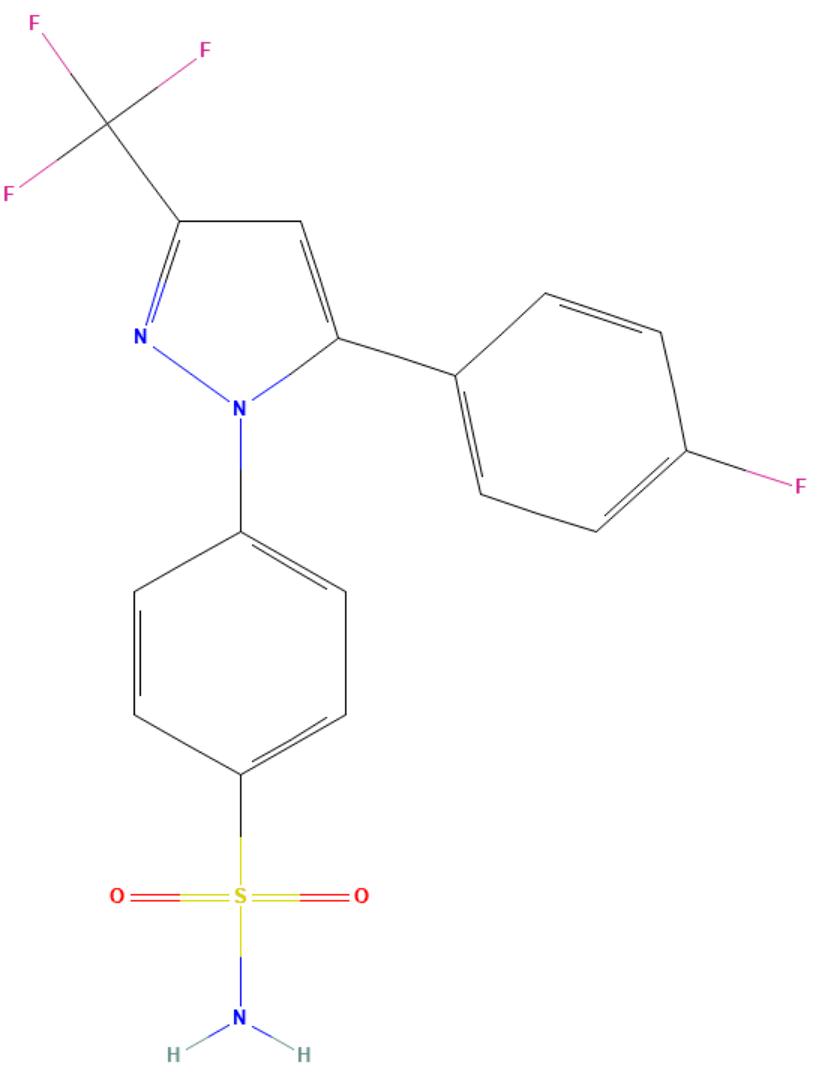
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
170569-88-7
Robenacoxib
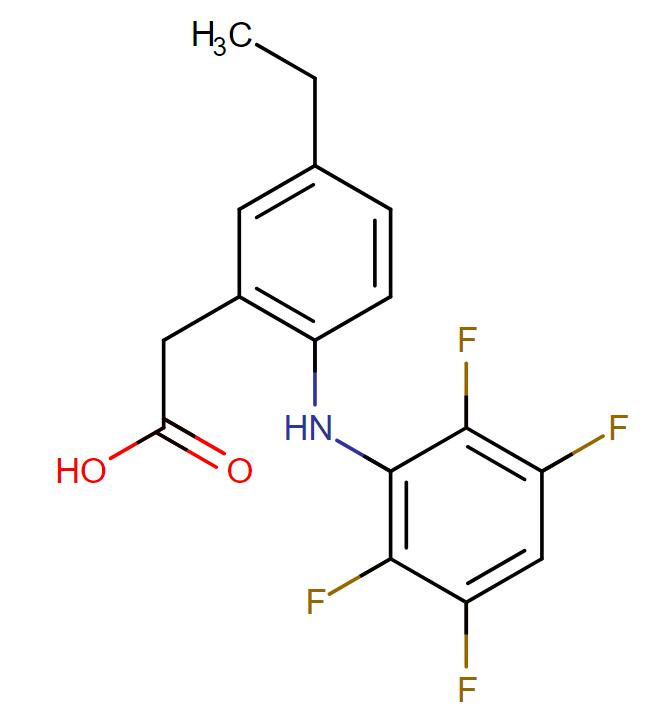
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, ASMF
CAS ID:
220991-32-2
Tildipirosin
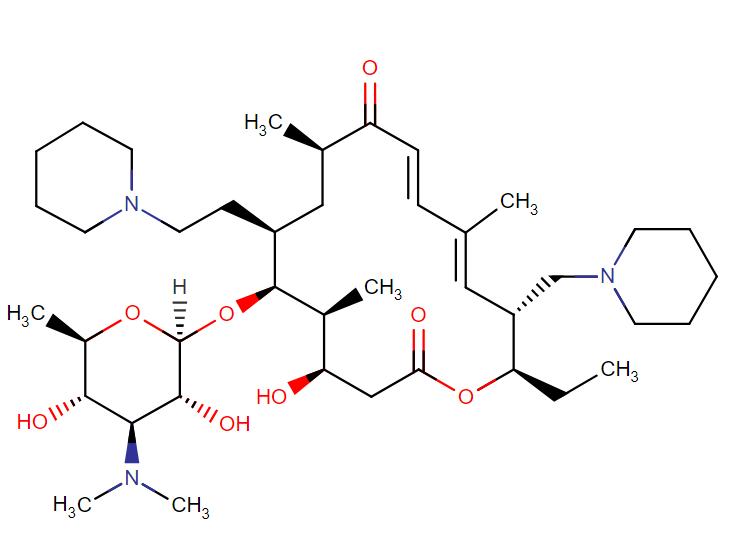
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
328898-40-4
Gamithromycin
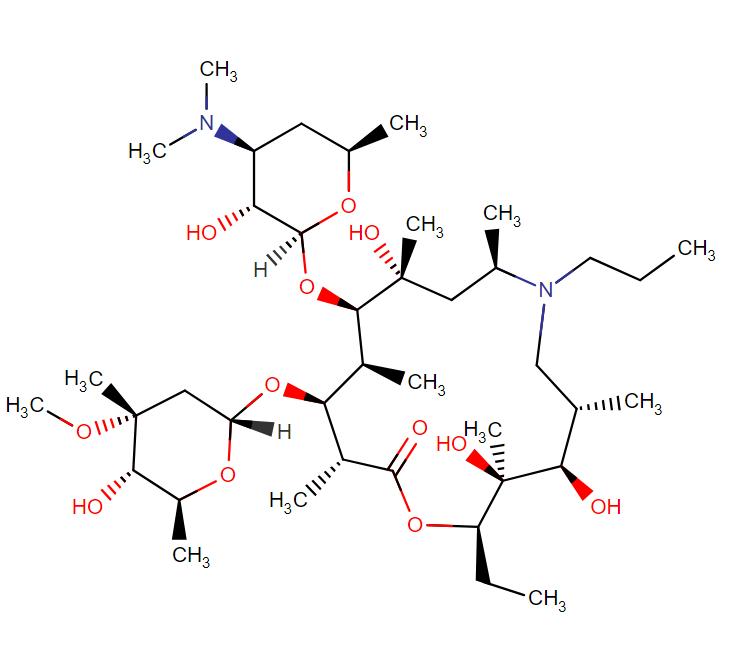
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
145435-72-9
Tulathromycin
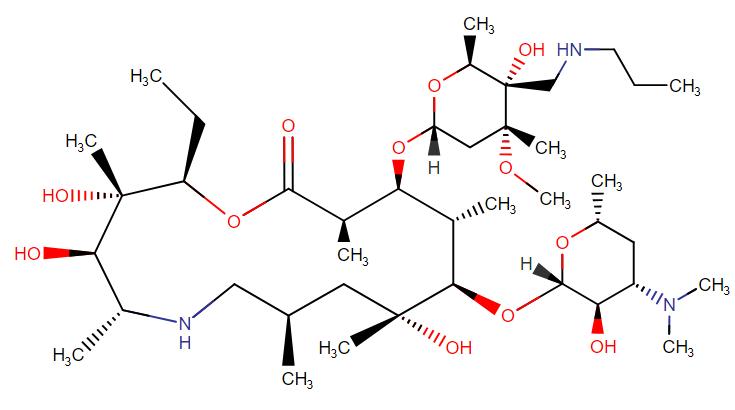
API Technology: Synthetic
Form: Powder
Development Status: Under development
Available regulatory filing:
Not filed
CAS ID:
217500-96-4
Butaphosphan
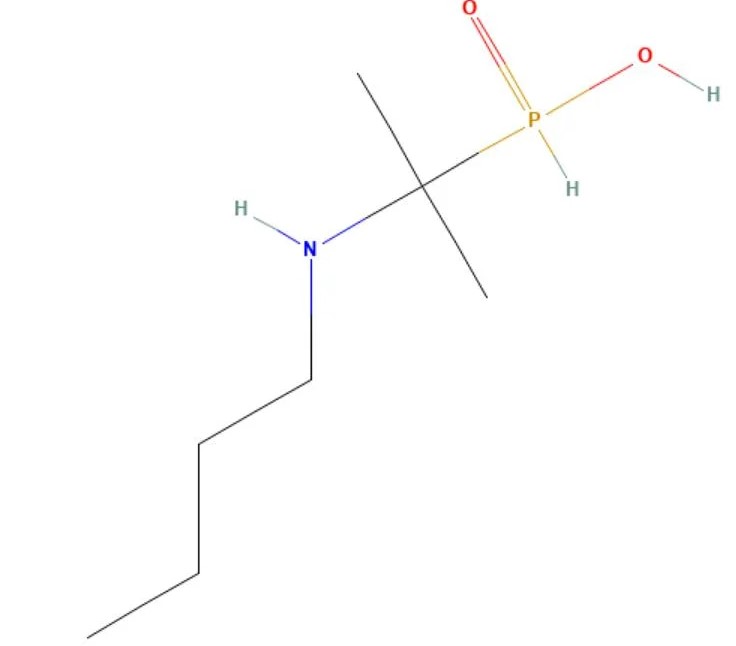
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, ASMF
CAS ID:
17316-67-5
Toldimfos sodium
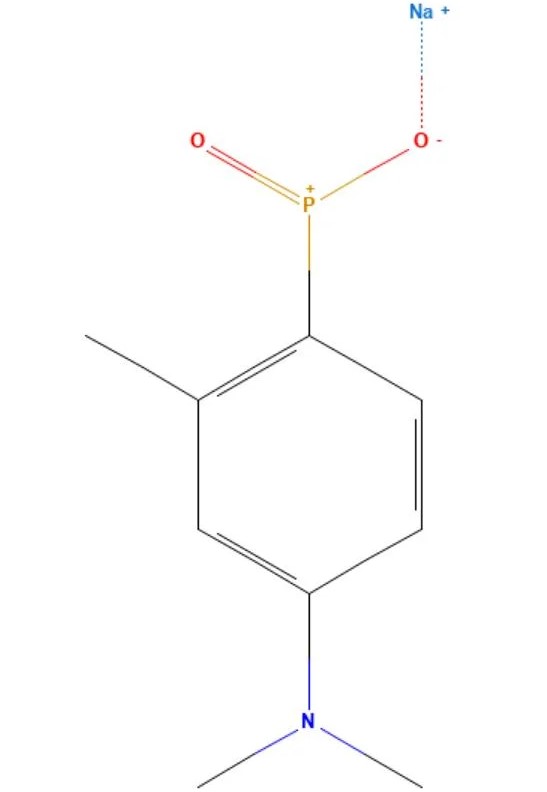
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
ASMF
CAS ID:
575-75-7
Zilpaterol
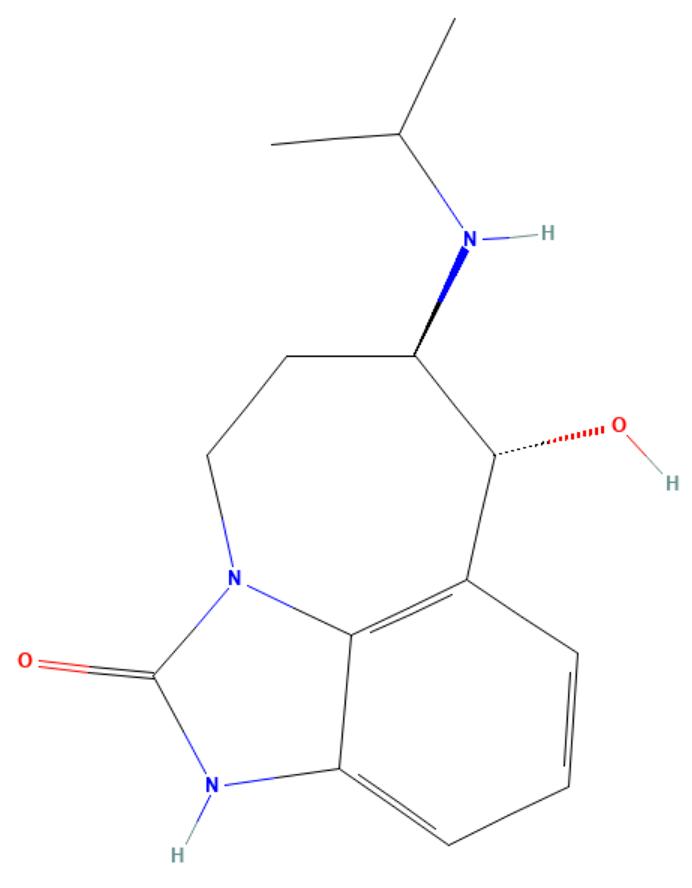
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
199520-06-8
Ractopamine HCl

API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF
CAS ID:
90274-24-1
Xylazine HCL
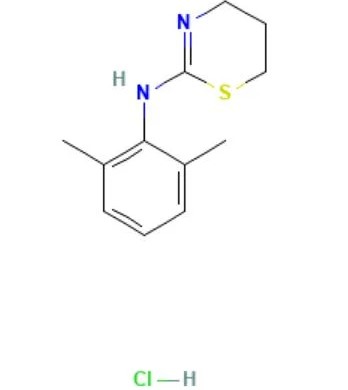
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
USVMF, CEP
CAS ID:
23076-35-9
Xylazine Base
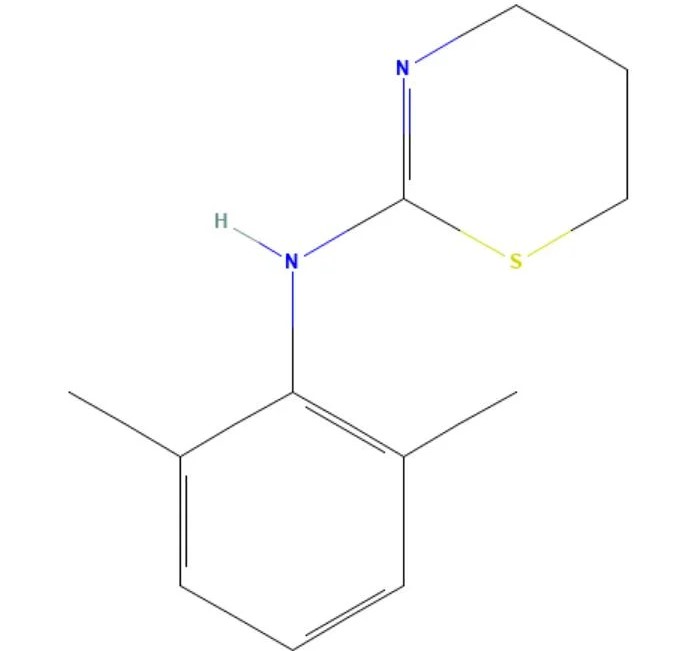
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
To be filed (under preparation)
CAS ID:
7361-61-7
Maropitant Citrate Monohydrate
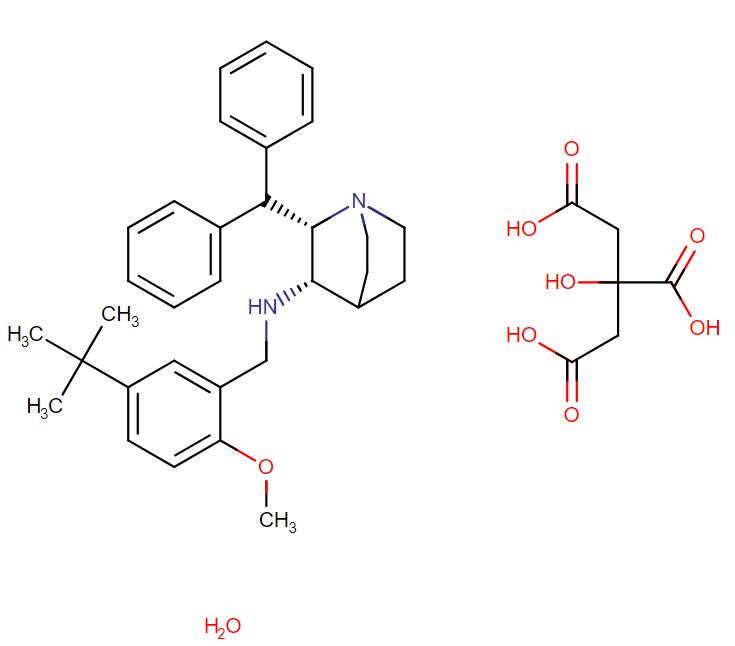
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Under planning
CAS ID:
359875-09-5
Maropitant base
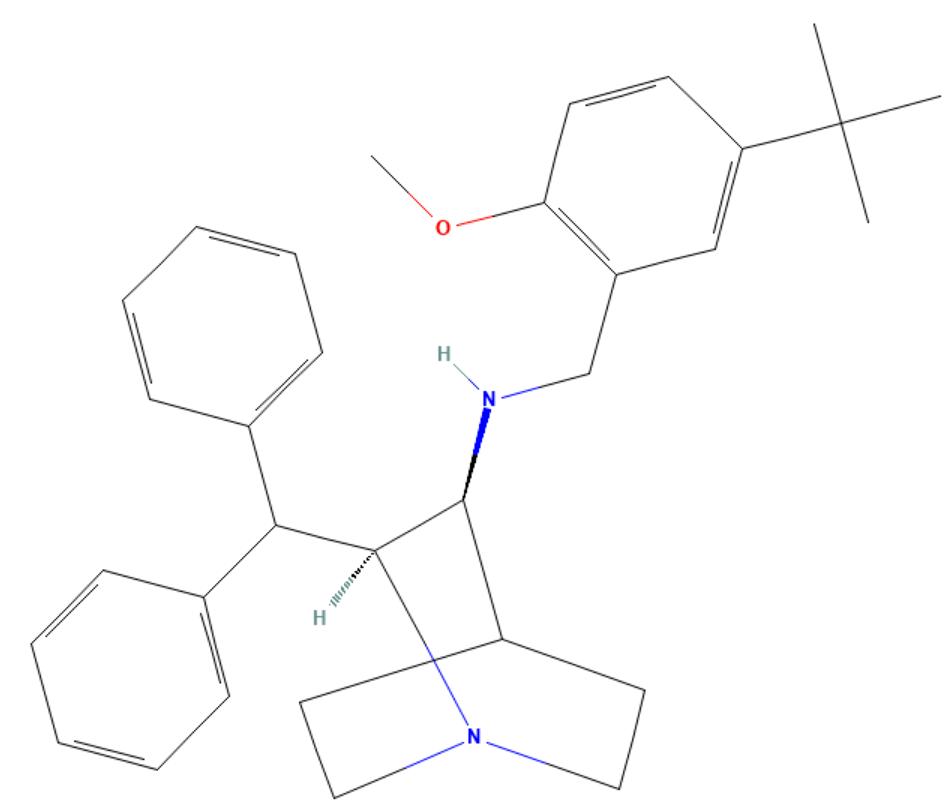
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Under planning
CAS ID:
147116-67-4
Oclacitinib Maleate
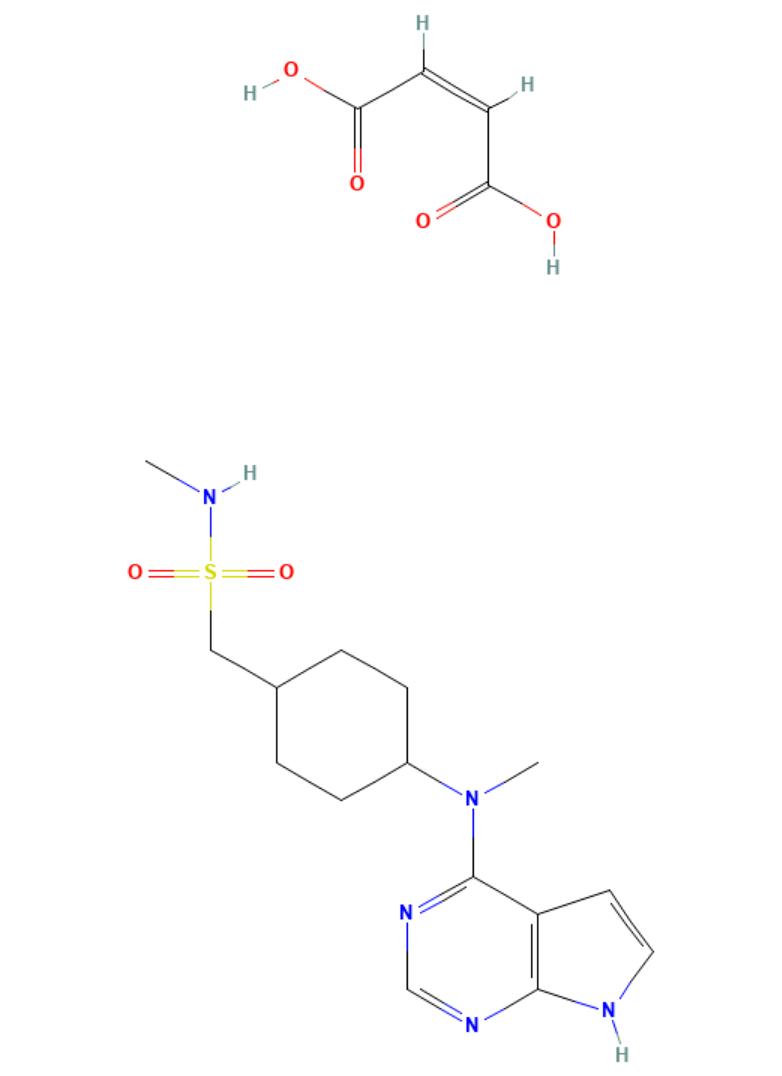
API Technology: Synthetic
Form: Powder
Development Status: Available
Available regulatory filing:
Not filed
CAS ID:
1208319-27-0
Pimobendan
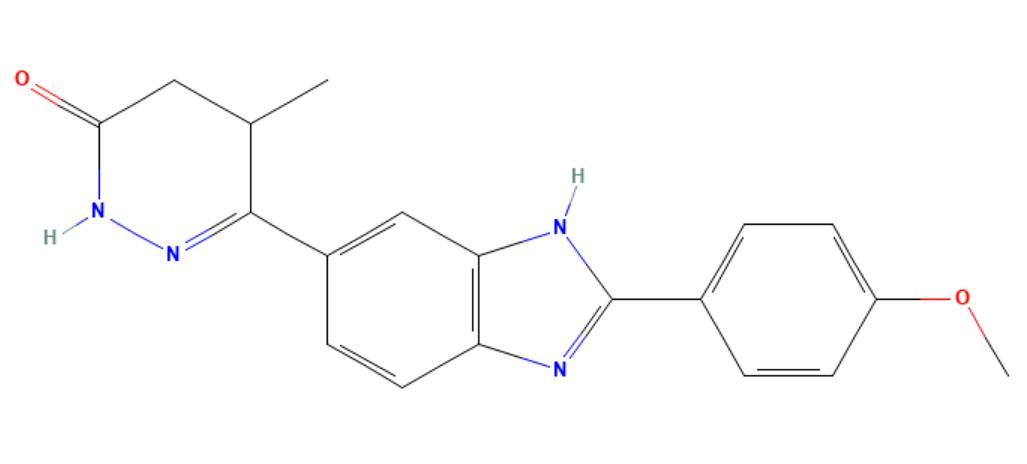
API Technology: Synthetic
Form: Powder
Development Status: Not Available
Available regulatory filing:
Under planning
CAS ID:
74150-27-9
Manufacturing
Capabilities
With a comprehensive portfolio of generic APIs and a strong track record in drug product development, SeQuent Scientific stands as one of the leading API manufacturers in the animal health sector, serving pharmaceutical companies in over 90 countries. Our state-of-the-art API manufacturing capabilities are complemented by formulation facilities that produce a wide range of dosage forms, ensuring end-to-end solutions for our global partners.
Our business is built on 30+ years of technical expertise in developing and manufacturing complex APIs, including anthelmintics, anti-infectives, and specialized veterinary compounds. We consistently deliver high-quality APIs and finished formulations to customers worldwide, enabling many to file successful dossiers and commercialize their products in international markets.
Our API manufacturing facilities adhere strictly to cGMP standards and are regularly audited by international regulatory authorities such as the USFDA, EU GMP, WHO–Geneva, COFEPRIS Mexico and so on.
Our facility in Mahad offers a reactor capacity of ~80 KL with 2 clean rooms, while our Vizag facility provides a reactor capacity of ~290 KL, equipped with 10 clean rooms.

100+
Markets globally

Alivira Animal Health’s API Research & Development Capabilities
As a global leader in active pharmaceutical ingredient (API) manufacturing, SeQuent
Scientific is a trusted partner for pharmaceutical companies across more than 90 countries. With strong R&D expertise in synthetic chemistry, analytical chemistry, and process engineering, we have built a robust portfolio of high-quality APIs, including niche segments such as anthelmintics, anti-infectives, and other veterinary APIs. Our advanced research capabilities enable us to meet the evolving needs of the animal health sector, while our commitment to quality ensures excellence in every stage of production. SeQuent Scientific continues to drive innovation in key therapeutic areas, including livestock, poultry, and companion animal health.
What we offer
India's Only USFDA approved veterinary API Facility
Highly Experienced technical team to support from Filing-to-launch

Backed by one of India's finest manufacturing facilities for seamless execution
Wide Range of Product Categories from Anthelmintic, Antiprotozoal, Beta Agonist NSAID etc


